I Do Not Recommend Amitriptyline (Elavil) for Digestive Disorders
The medication amitriptyline is commonly prescribed for people who suffer from reflux disorders. Amitriptyline is a tricyclic antidepressant that is known to have many different adverse side effects, some quite severe. Even one of the foremost researchers of silent reflux, Dr. Jamie Koufman, frequently prescribes and recommends the use of the antidepressant. I find it unsettling that she often recommends the use of the antidepressant in her practice even though it has numerous side effects, Especially since she cautions the use of proton pump inhibitor medications for silent reflux which also many side effects as well. So what is amitriptyline, why is it prescribed for reflux disorders, and why do I not recommend it’s use?
All About Amitriptyline (Elavil)
Amitriptyline is a tricyclic (its chemical structure contains three rings fused with a side chain) antidepressant medication that was discovered in 1960 and approved by the United Food and Drug Administration (FDA) in 1961. In 2016, it was the 88th most prescribed medication in the United States, with more than eight million prescriptions. It is a potent serotonin reuptake inhibitor, a moderate norepinephrine reuptake inhibitor, and does not strongly affect dopamine reuptake. A reuptake inhibitor is anything that reduces the body’s ability to reuptake neurotransmitters, increasing the amount of neurotransmitters in the synaptic cleft (space in between neurons). During reuptake, a transporter protein takes excess neurotransmitter molecules out of the synaptic cleft and puts them back into the neuron that released them reducing its bioavailability. The medication is also a histamine antagonist (H1 and H2), so it may reduce stomach acid production and blocks histamine’s effect on the brain as an alert neurotransmitter causing drowsiness. It also acts as an NDMA antagonist, decreases substance P, and activates adenosine A1 and opioid receptors, which reduces pain. Finally, amitriptyline inhibits sodium channels, calcium channel (L-type), and voltage-gated potassium channels (Kv1.1, Kv7.2, and Kv7.3), and therefore acts as a sodium, calcium, and potassium channel blocker (which explains its cardiovascular side effects because the heart requires these channels for proper conduction).1 2 3 4 5 6 7
The amitriptyline (Elavil) medication insert states its clinical pharmacology as the following:8
“Amitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system. Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of amitriptyline“
Amitriptyline is further metabolized in the liver by CYP2D6 and CYP2C19 to nortriptyline and has a half-life of twenty-five hours. If you have polymorphisms in CYP2D6 and CYP2C19 genes that encode those enzymes, you may need to discuss with your doctor about taking a smaller dose if you are using the medication because it will slow its metabolism. Furthermore, if people have multiple copies of these genes, they maybe ultrarapid metabolizers and require more of the medication for a therapeutic effect. Nortriptyline is a more potent and selective norepinephrine reuptake inhibitor than amitriptyline which causes greater norepinephrine concentrations within the synaptic cleft’s.9 10 11 12
What does the medication insert mention about the use of amitriptyline and CYP2D6?13
“The biochemical activity of the drug metabolizing isozyme cytochrome P4502D6 (debrisoquin hydroxylase) is reduced in a subset of the caucasian population (about 7 to 10% of Caucasians are so called “poor metabolizers”); reliable estimates of the prevalence of reduced P4502D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P4502D6, the increase in plasma concentration may be small, or quite large (8 fold increase in plasma AUC of the TCA)”.
“In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P4502D6 include some that are not metabolized by the enzyme(quinidine; cimetidine) and many that are substrates for P4502D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P4502D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the coadministration of TCAs with any of the SSRIs and also in switching from one class to the other.Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary)”.
“Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P4502D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P4502D6. Monoamine oxidase inhibitors; Guanethidine or similarly acting compounds; thyroid medication; alcohol, barbiturates and other CNS depressants; and disulfiram”.
What about amitriptyline and its interactions with other medications?14
“When amitriptyline is given with anticholinergic agents or sympathomimetic drugs, including epinephrine combined with local anesthetics, close supervision and careful adjustment of dosages are required. Hyperpyrexia has been reported when amitriptyline is administered with anticholinergic agents or with neuroleptic drugs, particularly during hot weather. Paralytic ileus may occur in patients taking tricyclic antidepressants in combination with anticholinergic-type drugs. Cimetidine is reported to reduce hepatic metabolism of certain tricyclic antidepressants, thereby delaying elimination and increasing steady-state concentrations of these drugs. Clinically significant effects have been reported with the tricyclic antidepressants when used concomitantly with cimetidine. Increases in plasma levels of tricyclic antidepressants, and in the frequency and severity of side effects, particularly anticholinergic, have been reported when cimetidine was added to the drug regimen. Discontinuation of cimetidine in well-controlled patients receiving tricyclic antidepressants and cimetidine may decrease the plasma levels and efficacy of the antidepressants. Caution is advised if patients receive large doses of ethchlorvynol concurrently. Transient delirium has been reported in patients who were treated with one gram of ethchlorvynol and 75 to 150 mg of amitriptyline hydrochloride.“
Amitriptyline is used for the following medical conditions:15 16 17 18 19 20 21 22
- Anxiety
- Bipolar disorder
- Cyclic vomiting syndrome (I would actually argue it was the CoQ10 used in the study which increased mitochondrial function reducing the symptoms of cyclic vomiting syndrome and not the amitriptyline)
- Eating disorders
- Depression
- Fibromyalgia
- Insomnia
- Irritable bowel syndrome (diarrhea)
- Major depression disorder
- Migraines
- Neurogenic cough
- Nocturnal enuresis (bed wetting)
- Pain (mostly nerve related, sometimes used for arthritic pain and abdominal pain)
- Parkinson’s disease (anticholinergic medication)
- Psychosis
- Post-traumatic stress disorder
- Schizophrenia
- Sphincter of Oddi dysfunction
- Tension headaches
- Visceral hypersensitivity (mainly in reflux disorders)
- Vagal neuropathy
Amitriptyline is used in reflux disorders to help prevent neurogenic cough, improve vagal nerve tone (to help esophageal motility and sphincter closure), relieve globus pharyngeus, and to reduce the pain associated with reflux (visceral hypersensitivity).23 24 25 26
So what does the medication insert say is the official labeled use for amitriptyline?27
“For the relief of symptoms of depression. Endogenous depression is more likely to be alleviated than are other depressive states.”
Well, the medication is being used for a heck of a lot more medical conditions than depression. What are the numerous side effects for amitriptyline?28



Wow, there are numerous listed side effects, and the medication has significant withdrawal potential. Some of the listed side effects that occur frequently include dry mouth, drowsiness, weight gain, sexual dysfunction, arrhythmia, elevated blood pressure, and digestive issues (including constipation [slows down motility] and worsening reflux [loosens the esophageal sphincters]). The medication has also been associated with causing coenzyme-Q10 deficiency (causing mitochondrial toxicity), so if you decide to take it you should talk to your doctor about supplementing with ubiquinol while you are on the medication. Are there any warnings in the drug insert associated with the use of the medication?29 30

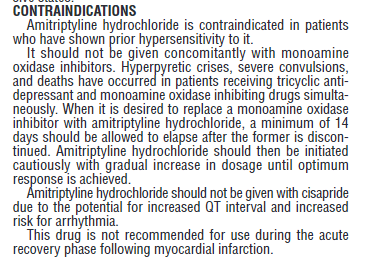
So the use of amitriptyline is cautioned in children, adolescents, and young adults and is not approved in pediatric patients. So what is the risk of suicide for children under the age of eighteen who are using amitriptyline versus a placebo?31
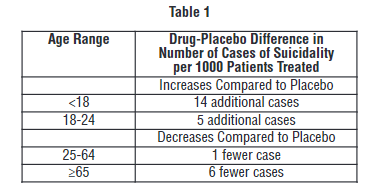
Fourteen additional suicides per thousand patients treated under the age of eighteen. In addition:32
“It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.”
Its use is also cautioned in people with cardiovascular disease, people prone to QT arrhythmia’s, and people who have recently suffered a heart attack. Finally, its use is cautioned for people with bipolar disorder (it may worse mania and may trigger suicidal tendencies), seizure disorders, and people with angle-closure glaucoma.33
Why I Do Not Recommend the Use of Elavil for Reflux Disorders
Did you skip to this part of the blog and bypassed the numerous side effects associated with amitriptyline (Elavil)? If you did then go back and read the first section of my blog to discover why I would never recommend its off label non-approved use for reflux disorders because of its side effect profile. However, are there other reasons why I would not recommend the use of amitriptyline (Elavil) for reflux disorders?
- Though amitriptyline (Elavil) may reduce pain and visceral hypersensitivity, however, it does not work on the root causes of your reflux. Therefore for most people using it either the reflux continues, and they do not feel it or when they stop the medication reflux reoccurs because the underlying problem is not fixed. For some, the medication only reduces reflux symptomology for a specific period of time, and they either increase the dosage to try to regain relief or discontinue. Many people report on reflux forums and groups that when they stop the medication, their symptoms generally return, sometimes being unbearable.34
- Amitriptyline (Elavil) weakens the upper esophageal sphincter and lower esophageal sphincter by relaxing the smooth muscle which for most people worsens their reflux even though they do not feel it occurring.35 36
- Amitriptyline (Elavil) slows down gastric emptying (except in small doses, 12.5 milligrams three times daily for example) and motility (relaxes the smooth muscles that contract for peristalsis) and is prone to cause constipation. Constipation and slow gastric emptying can increase abdominal pressure, which can lead to developing a hiatal hernia and reflux. Delayed gastric emptying and constipation can lead to increased fermentation and gas production from food remaining in the digestive tract for too long, causing gas and bloating. Increase in gas and bloating can lead to increased abdominal pressure as well.37 38 39
- Amitriptyline (Elavil) reduces stomach acid production as a H2 antagonist which can lead to or enhance dysbiosis of the stomach and can lead to maldigestion causing increased fermentation and gas production from an elevated stomach pH.
- Amitriptyline (Elavil) has too many side effects for its off-labeled use in reflux disorders. I believe that it would provide too many risks and not enough benefits for its use.40
- There are many things you can try to improve vagal tone without having to use the medication.
- Wirfs, Mari. The PA’s Complete Guide to Prescribing Drug Therapy, Springer Publishing Company; 2019 edition, April 17, 2018 ↩
- Sneader, Walter. Drug Discovery a History. Chichester, John Wiley & Sons, 2005 ↩
- https://www.ncbi.nlm.nih.gov/pubmed/17456683 ↩
- https://pdsp.unc.edu/databases/pdsp.php?knowID=0&kiKey=&receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testLigandDD=&testFreeRadio=testFreeRadio&testLigand=amitriptyline&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query ↩
- https://www.ncbi.nlm.nih.gov/pubmed/39202 ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5489810/ ↩
- Porter, Robert. The Merck Manual, Merck; 19 edition, July 20, 2011 ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- Wirfs, Mari. The PA’s Complete Guide to Prescribing Drug Therapy, Springer Publishing Company; 2019 edition, April 17, 2018 ↩
- Porter, Robert. The Merck Manual, Merck; 19 edition, July 20, 2011 ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://deepblue.lib.umich.edu/bitstream/2027.42/109971/1/cptclpt20132.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- https://www.ncbi.nlm.nih.gov/pubmed/21414249 ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2825193/ ↩
- Porter, Robert. The Merck Manual, Merck; 19 edition, July 20, 2011 ↩
- Wirfs, Mari. The PA’s Complete Guide to Prescribing Drug Therapy, Springer Publishing Company; 2019 edition, April 17, 2018 ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594960/ ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027558/ ↩
- Koufman, Jamie. The Chronic Cough Enigma, Katalitix, February 11, 2014 ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594960/ ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027558/ ↩
- Koufman, Jamie. The Chronic Cough Enigma, Katalitix, February 11, 2014 ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3831229/ ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- https://www.ncbi.nlm.nih.gov/pubmed/22118833 ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/http://www.sniflmd.com/pdf/Extra-esophageal%20Reflux%20and%20the%20Ear,%20Nose%20and%20Thraot%20%202017%20.pdf ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/http://www.sniflmd.com/pdf/Extra-esophageal%20Reflux%20and%20the%20Ear,%20Nose%20and%20Thraot%20%202017%20.pdf ↩
- https://www.ncbi.nlm.nih.gov/pubmed/28258931 ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3897125/ ↩
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3710425/ ↩
- chrome-extension://oemmndcbldboiebfnladdacbdfmadadm/https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf ↩
I do not recommend the use of Amitriptyline (Elavil) in reflux disorders instead, I recommend that you attempt to discover the root causes of your reflux and try to relieve them instead. If you are suffering from reflux contact me for coaching and we can see what we can do to get you better!






Good article but what would you actually recommend to someone who is suffering horribly with this condition?
I had an endoscopy and the esophagus biopsy showed mild gerd. I feel like someone is squeezing my throat all day and it’s overwhelming me.
I have colitis and had my large intestine removed in 2007. I never get actual heartburn but do get occasional chest pain. My diet is so bland, I have zero spicy, fried, fatty, mint, carbonated, caffeine or fast food, I am underweight if anything, I elevate my head in bed, and I’ve had food allergy testing and am fully compliant. I eat only healthy organic food, and no wheat, corn, junk etc.
I am at a complete loss and can’t take much more of this hoarse, tight throat 24/7.
Thank you soooooooooooo much for your guidance.
Suffering GERD/IBS for a year, nausea,fatigue, weight loss. Seen 2 gatric surgeons & had Gastroscopy/colonoscopy showed nothing. Tried various diets & alternative stuff & on 40 mg nexium.
Sydney Clinic has recommended trying 10mg Amitriptyline a day to assist motility – they say it works for some people.
Interesting and thorough article.
I’m doing research to try to understand if nortriptyline (the active metabolite of amitriptyline) may be causing my “mild esophageal dysmotility with mildly reduced and weakened primary peristalsis” which was discovered as a result of a Barium swallow imaging test.
So far, my research indicates that nortriptyline’s effect on smooth muscles is to cause relaxation and slow propagation. However, I have not been able to find any research that is exactly on point regarding nortriptyline’s effect on peristalsis.
Are you aware of this possible effect? What are your thoughts?
Thanks,
Don Holtz
Vancouver, BC, Canada
Yes, is my belief that it would weaken the LES and reduce esophageal dysmotility. No studies to my belief.